Hydrogel-Based Microfluidic Cell Culture 99
The biocompatibility of PDMS can be increased by serially washing the PDMS
to extract uncrosslinked oligomers, solvents, and catalyst which can leach out,
reducing cell viability in cell culture devices.
49
Further work is needed to inves-
tigate whether PDMS has a significant absorption for the drugs or other elements
from the culture media. The uptake of small molecules into PDMS microstructures
or release of uncured oligomers
50−52
can lead to adverse results for cell culture,
drug screening and discovery, or cytotoxicity assays. In this case, the culture
chambers may need to be coated in order to reduce uptake of small molecules
into PDMS microstructures and leaching of uncured oligomers.
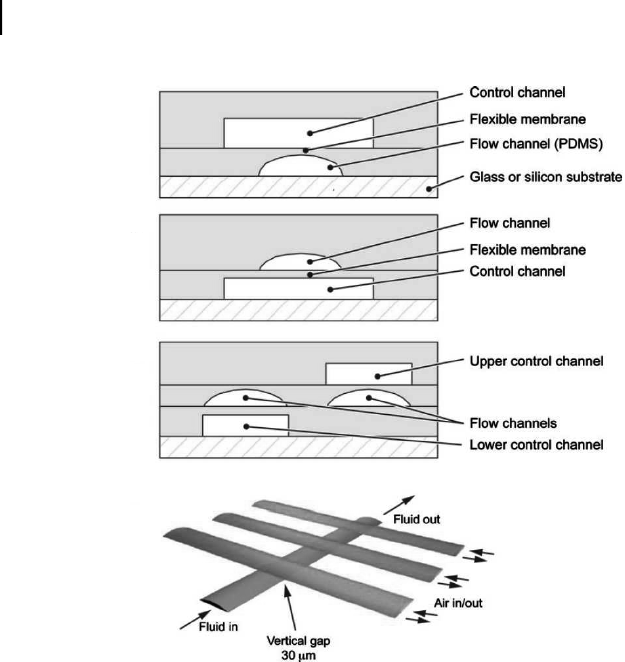
PDMS has been used to make valves, both as an elastomeric diaphragm
integrated into glass or silicon devices, and also as membranes in multilayer PDMS
structures fabricated using soft lithography.
53
In these structures, one layer of the
device contains the control channels and the other layer contains the flow channels.
The membrane where the control channel and flow channel intersect is deflected
into the flow channel, often pneumatically, to effect valving (Fig. 5.8). Peristaltic
pumps, created using three such membrane valves in a row, can be used to achieve
pumping rates as high as 2 cm/s at 100 Hz. Microfluidic valves can also be used
to create mixers by putting peristaltic pumps in a closed loop.
In cases where controlling liquid loss through evaporation is critical, the PDMS
can be coated with parylene to reduce gas and moisture permeability. Parylene is
a biocompatible polymer that is conformally deposited inside the channel. The
deposition is done from the vapor phase at room temperature to give a thin-film
coating that has low residual stress. Parylene films are pinhole-free even at low
thickness and have low moisture permeability. Parylene coatings have already
been used to reduce the sample evaporation and protein adsorption in polymerase
chain reaction (PCR) chambers,
45
as well as control moisture loss in microfluidic
cell culture devices.
54
Alternatives to PDMS as a structural material include glass,
polystyrene, and polyimide. Other solvent-resistant materials include microfluidic
channels molded from thiolene or structures molded in photocurable teflon. Of
these materials, however, the production of prototypes and test devices with
multiple layers/heights and valving functionality using PDMS is still the most
cost effective and time efficient in most cases.
5.4 HYDROGEL-BASED MICROFLUIDIC CELL CULTURE
Rather than continuing to miniaturize conventional cell culture systems, such as
moving from 96-well to 384- or even 1536-well plates, moving to microfluidic
systems can offer advantages such as fluid handling and transport. For systems
with integrated sensors, scaling down the physical dimensions can lead to im-
proved sensor signal/noise ratio and response time.
55
Microfabricated systems
can offer lower power consumption, lower fabrication costs, and smaller footprint
than conventional systems. Controlled spatial and temporal gradients are easily
produced using microfabricated systems
56
(Fig. 5.9).
SO13997_text.indd 107SO13997_text.indd 107 26/01/2011 3:50 PM26/01/2011 3:50 PM
100 M. C. W. Chen and K. C. Cheung
(b)
(c)
(d)
(a)
Figure 5.8. (a) A two-layer polydimethylsiloxane (PDMS) push-down microfluidic valve.
An elastomeric membrane is formed where the flow channel is positioned orthogonal to
the control channel directly above. Fluid flow is out of the page. (b) A two-layer PDMS
push-up microfluidic valve where a control channel lies orthogonal to and below the flow
channel. (c) A three-layer device with both push-up and push-down valves. (d) Schematic
of a linear peristaltic pump using three membrane valves in a series.
53
For color reference,
see page 262.
Shear stress in the culture environment can also affect cell differentiation.
57
Although a wide range of fluid velocities and shear stresses are seen in different
areas of the body, shear stress in perfusion culture, in which cells adhere to the
bottom of a microfluidic culture channel, may not be desirable for all cell types.
Shear stress has been found to induce tumor cell cycle arrest, thus affecting tumor
cell sensitivity to anticancer agents.
58
Even low perfusion rate in microfluidic cell
culture has been found to affect growth kinetics and morphology.
59
By reducing
the flow so that shear stress is on the order of 0.01 Pa, which approximates many
in vivo conditions, a continuous flow environment can be used to maintain cell
viability for long-term culture.
60,61
Encapsulation within a gel may be one way to protect cells from shear stress
due to the perfusion of culture medium at higher flowrates. Perfusion culture can
be combined with 3-D culture matrices within microfluidic devices.
62
In this case,
SO13997_text.indd 108SO13997_text.indd 108 26/01/2011 3:50 PM26/01/2011 3:50 PM
Get Biomaterials for MEMS now with the O’Reilly learning platform.
O’Reilly members experience books, live events, courses curated by job role, and more from O’Reilly and nearly 200 top publishers.

