106 M. C. W. Chen and K. C. Cheung
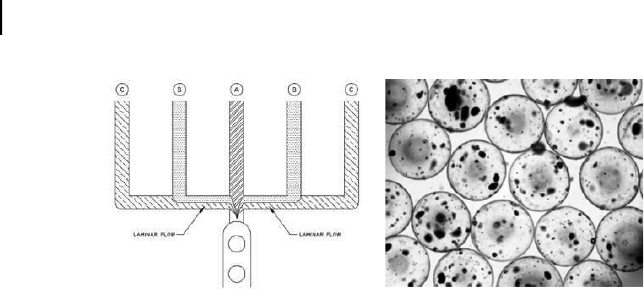
Figure 5.14. (left) The shielded junction employed to generate alginate microspheres.
Aqueous sodium alginate mixed with CaCO3 and cells is introduced into the central
channel (A). Sunflower oil mixed with acetic acid is supplied to the outermost channels (C).
Sunflower oil is supplied to the intermediate channels (B) to act as a shield preventing the
alginate solution from coming into contact with the acidified oil flow. Between channels
B and A the two oils flow in a laminar fashion, with minimal diffusion of H+ into the
protective sunflower oil. After droplet formation at the junction, H+ diffuses into the
alginate droplet, thus liberating Ca2+ from CaCO3, which causes gelation of the alginate.
(right) Light microscope image of encapsulated PC12 cells, showing small cell clumps
present after encapsulation.
82
U-2 OS cell lines.
82
A droplet-based microfluidic system has also been used to form
alginate beads with entrapped breast tumor cells. The alginate environment per-
mitted cell proliferation and the formation of multicellular spheroids. The dose-
dependent response of the tumor spheroids to doxorubicin, and anticancer drug,
showed multicellular resistance compared to conventional monolayer culture.
83
5.4.4 Other Configurations
A photopolymerizable polyethylene glycol (PEG) was used in combination with
dielectrophoresis (DEP) to trap cells in controlled, reproducible organizations.
84
The ability to pattern cell clusters with varying size and spacing showed that
microscale organization and tissue architecture can affect cell behavior.
A combination of micropillars and gel encapsulation has been used in a
3-D perfusion system
85
by Toh et al. In this work, the cells were immobilized
using the micropillar array, and then the cells were stabilized by a coacervated
methylated collagen/HEMA-MMA-MAA terpolymer. Their work has also used
polyethyleneimine-hydrazide as an inter-cellular linker, instead of the coacervated
hydrogel, to created a “gel-free” system.
86
Soft lithography and replica molding has been used to cast 3-D gel objects from
collagen, Matrigel, and agarose.
87
The cell densities in the gel modules ranged
from 10
8
–10
9
cells/cm
3
, which approaches the density in tissues. The gel objects
had lateral dimensions from 40 to 1000 μm.
On-chip gelation of NiPAAm has been achieved using microheaters to
encapsulate single cells which have been positioned using optical tweezers.
88
Since
NiPAAm is a thermoreversible gel, the entrapped cell can be released by turning
SO13997_text.indd 114SO13997_text.indd 114 26/01/2011 3:50 PM26/01/2011 3:50 PM
Get Biomaterials for MEMS now with the O’Reilly learning platform.
O’Reilly members experience books, live events, courses curated by job role, and more from O’Reilly and nearly 200 top publishers.

