
197
13
Nuclear Energy
Introduction
Nuclear energy is energy that is obtained from the nucleus of an atom. The
energy is released from an atom through one of two processes: (1) nuclear
fusion or (2) nuclear ssion.
Energy is released in nuclear fusion when atoms are combined or fused
together (fusion). This is how energy is generated in the sun. In nuclear s-
sion, energy is released when atoms are split apart. Nuclear ssion is the only
method currently used by nuclear plants to generate electricity. However, the
terms nuclear fusion and nuclear ssion are referred to synonymously as
nuclear energy. Both involve changes in (nuclear) mass.
Regarding ssion, uranium is the heaviest of the 92 naturally occurring
elements. Since it is also one of the few elements that is easily ssioned, it
is the fuel of choice used by today’s nuclear power plants. This element was
formed when the Earth was created and is usually found in rocks. Rocks that
contain signicant quantities of uranium are referred to as uranium ore, or
pitchblende. Two forms (isotopes) of uranium are found in these rocks: ura-
nium-235 and uranium-238. These three-digit numbers refer to the number of
neutrons and protons in each atom. Uranium-235 is the form normally used
for energy production because, unlike uranium-238, the nucleus splits more
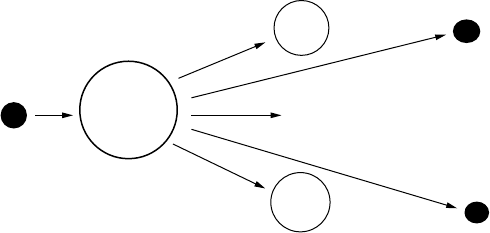
easily when bombarded by a neutron. During ssion, the uranium-235 atom
absorbs a bombarding neutron, causing its nucleus to split apart into two
atoms of lighter weight. (See Figure13.1.) The ssion reaction simultaneously
releases energy as both heat and radiation. It also releases more neutrons.
These released neutrons go on to bombard other uranium atoms, and the
process repeats itself. This repetitive process is referred to as a chain reaction.
When a uranium atom is split apart (ssioned), the mass of the fragments
is less than the mass of the original atom. The energy corresponding to this
loss of mass is dened as ssion energy. It is represented in equation form by
Einstein’s equation
[1]:
=E mc
2
(13.1)
198 Energy Resources: Availability, Management, and Environmental Impacts
where (in SI units)
E = joules (J)
m = kilograms (kg)
c = meters/second (m/s); the velocity of light, 3 × 10
8
m/s
One can show that each kilogram of mass converted liberates approxi-
mately 10
17
J. This thermal energy is enough to run a large turbine generator
(a 1-GW unit) for a year at 35 percent thermal efciency and is equivalent to
the energy possessed by 2.5 million tons of coal.
Similarly, if two or more light elements (e.g., hydrogen) are combined
(fused) to form a heavier one, the resulting mass of the atom is less than the
sum of the two original atoms. The difference (or loss) in mass is converted
to energy, as with the fusion process. This energy is dened as fusion energy.
The peaceful use of nuclear energy involves the following process. Nuclear
energy is generated by the aforementioned splitting of uranium atoms. The
energy in the form of heat from this ssion process is used to drive a turbine
to generate electricity. The operation of a nuclear reactor and the related elec-
tric generating equipment is only one part of an interconnected set of indi-
vidual processes. These processes include mining, milling, and transporting
uranium; enriching uranium and packing it in appropriate form; designing
and constructing the reactor and auxiliary equipment; and treating and dis-
posing of spent fuel. All these activities thus require several sophisticated
and interactive industrial processes that involve many separate pieces of
equipment requiring many specialized skills. Additional details are pro-
vided in the next section.
When commercial use of nuclear power was rst developed (in the late
1950s—see also the next section), many thought it would become the great
savior of human civilization, providing a source of clean, limitless electric-
ity that would replace fossil fuels in the future. Unfortunately, that has not
come to fruition; simply put, nuclear power has yet to achieve that promise,
and concerns to be discussed later have severely hampered the development
Neutron
Neutron
Energy
Atom Splits
Uranium
235
Lighter
Element
Lighter
Element
Neutron
Fission
FIGURE 13.1
The ssion process.
Get Energy Resources now with the O’Reilly learning platform.
O’Reilly members experience books, live events, courses curated by job role, and more from O’Reilly and nearly 200 top publishers.

