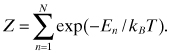2
Basic Quantum Statistical Mechanics
2.1 ELEMENTARY STATISTICAL MECHANICS
If a system in equilibrium can be in any of N states, then the probability of the system having energy ![]() is given by
is given by ![]() , where the partition function Z is defined by [1]
, where the partition function Z is defined by [1]
Here, ![]() is a Boltzmann constant, and T is a temperature. For quantum mechanical system of states
is a Boltzmann constant, and T is a temperature. For quantum mechanical system of states ![]() , where
, where ![]() denotes the state with energy
denotes the state with energy ![]() , the expected value of the quantum mechanical operator A is defined by
, the expected value of the quantum mechanical operator A is defined by
(2.2)
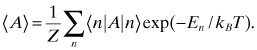
This fundamental law is the essence of statistical mechanics, with the concept of thermal equilibrium and temperature. If a system is very weakly coupled to a heat bath at a given temperature, if the coupling is indefinite or not known ...