10
Group 1 (IA) The alkali metals
Alkaline metals are Li, Na, K, Rb, Ss and Fr. General properties of alkali metals are given below:
Table 10.1
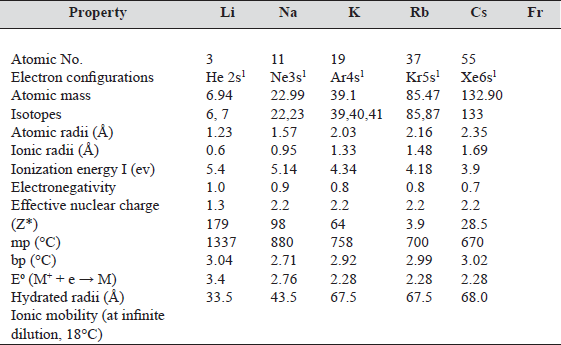
All the metals cyrstallize with body centered cubic (BCC) lattice, i.e., coordination number is eight. This structure is adopted due to large atomic radius.
Figure 10.1
It is not the closest packed structure.
As there is only one valence electron per atom, the binding energy in the metal lattice is relatively weak. The metals are, thus, very soft and have low melting points. (A Na — K alloy with 77.2% ...
Get Basic Concepts of Inorganic Chemistry, 2nd Edition now with the O’Reilly learning platform.
O’Reilly members experience books, live events, courses curated by job role, and more from O’Reilly and nearly 200 top publishers.

