16
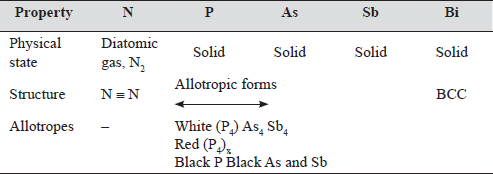
Group – 15 (VA) N, P, As, Sb, Bi
Elements of this group are called Pnicogens. (Greek, pnigmos-suffocation, many compounds of the group elements have suffocating oduour, so this name). There is a transition from non-metal to metal.
Nitrogen and Bi do not show allotropy whereas P, As and Sb have allotropes.
Table 16.1
Note:
- Nitrogen is diatomic gas, N2 at NTP. It is due to very effective (p — p)π bonding in N2.
N 2s22p3 i.e., three unpaired electrons in p — orbitals. There are one σ and two π bonds in N2. Nitrogen is sp-hybridized.
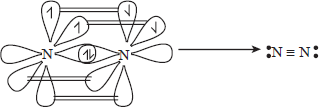
Figure ...
Get Basic Concepts of Inorganic Chemistry, 2nd Edition now with the O’Reilly learning platform.
O’Reilly members experience books, live events, courses curated by job role, and more from O’Reilly and nearly 200 top publishers.

