19
Group — 18, The Noble Gases
Helium, Ne, Ar, Kr, Xe and Rn are noble gases and elements of group 18 (or zero group). They are minor constituents of the atmosphere. Sir William Ramse first isolated Ne, Ar, Kr and Xe. He also established He. It is found in stars, in radioactive minerals and some natural gasses. Its origin is entirely from the decay of thorium and uranium isotopes, which emit α-particles. The last member of the group Rn is radioactive (all isotopes). These gases are obtained by fractionation of liquid air.
Properties
Table 19.1
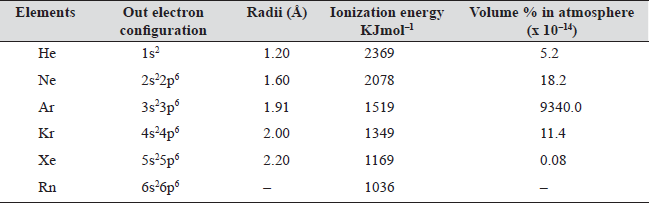
Note
- Ar is most abundant and Kr is least
Atomicity
All noble gases are monoatomic. It is due to closed-shell ...
Get Basic Concepts of Inorganic Chemistry, 2nd Edition now with the O’Reilly learning platform.
O’Reilly members experience books, live events, courses curated by job role, and more from O’Reilly and nearly 200 top publishers.

