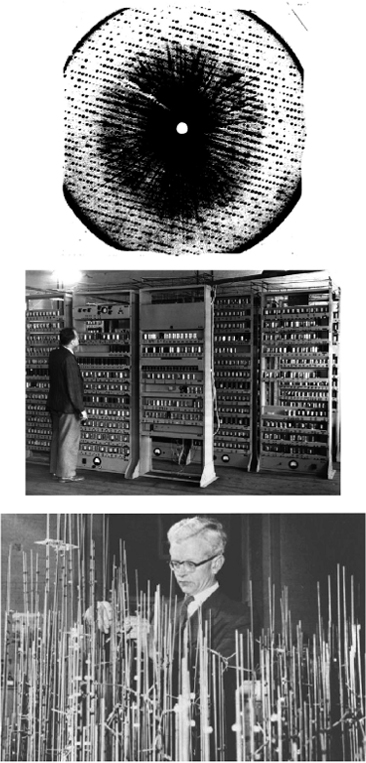
Beginning in the 1940s, Max Perutz and John Kendrew realized the goal of determining the structure of globular proteins by solving the structure of hemoglobin and myoglobin. In recognition of this work, they shared the Nobel Prize in Chemistry in 1962. (top) X-ray precession photograph of a myoglobin crystal (from ![]() http://www.nobel.se/chemistry/laureates/1962/kendrew-lecture.pdf). Kendrew studied myoglobin from the sperm whale (Physeter catodon), and incorporated a heavy metal by the method of isomorphous replacement. He could then bombard the crystals with x-rays in order to obtain an x-ray diffraction pattern (such as that shown here) with which to deduce the electron density throughout the crystal. This required the analysis of 25,000 reflections. (middle) Perutz and Kendrew used the EDSAC I computer (introduced in 1949 and shown here (from
http://www.nobel.se/chemistry/laureates/1962/kendrew-lecture.pdf). Kendrew studied myoglobin from the sperm whale (Physeter catodon), and incorporated a heavy metal by the method of isomorphous replacement. He could then bombard the crystals with x-rays in order to obtain an x-ray diffraction pattern (such as that shown here) with which to deduce the electron density throughout the crystal. This required the analysis of 25,000 reflections. (middle) Perutz and Kendrew used the EDSAC I computer (introduced in 1949 and shown here (from ![]() http://www.cl.cam.ac.uk/Relics/jpegs/edsac99.36.jpg). This computer was essential to interpret the diffraction patterns. For a simulator that shows the capacity of the EDSAC machine, see
http://www.cl.cam.ac.uk/Relics/jpegs/edsac99.36.jpg). This computer was essential to interpret the diffraction patterns. For a simulator that shows the capacity of the EDSAC machine, see ![]() http://www.dcs.warwick.ac.uk/~edsac/. (bottom) Photograph ...
http://www.dcs.warwick.ac.uk/~edsac/. (bottom) Photograph ...
Get Bioinformatics and Functional Genomics, Second Edition now with the O’Reilly learning platform.
O’Reilly members experience books, live events, courses curated by job role, and more from O’Reilly and nearly 200 top publishers.

