120 V. Bazargan and B. Stoeber
consisting of PEO-PPO block copolymers belong to this group and are commer-
cially available and commonly used to form TRFs.
6.4.2 Properties of Pluronic Solutions
6.4.2.1 Phase Transition of Pluronic Solutions
Pluronics are biocompatible symmetric triblock copolymers of poly(ethylene
oxide)
x
-poly(propylene oxide)
y
-poly(ethylene oxide)
x
;P(EO)
x
–P(PO)
y
–P(EO)
x
.
These amphiphilic copolymers have hydrophilic EO
(x)
and hydrophobic PO
(y)
units where x and y can take on specific values depending on the particular kind
of Pluronic. Aqueous Pluronic solutions undergo reversible gel formation at a
specific critical solution temperature that depends on the polymer concentration,
its molecular weight and the polymer architecture.
29,32,90,91,93−95
The sol-gel tran-
sition of Pluronic solutions occurs through three dimensional packing of polymer
micelles into a lattice, to achieve a hydrophilic/hydrophobic balance.
34
Above the critical micelle concentration (CMC) the polymers aggregate into
multi molecular structures with a hydrophobic central core (PO) and a hydrophilic
shell(EO)facingtheexternalmedium.
96,97
At higher concentrations, above the
critical gel concentration (CGC) the micelles can take on a cubic lattice structure.
34
Elevated temperatures lead to dehydration of the hydrophobic core and also
increase the hydrophobicity of the hydrophilic blocks, which eventually leads to
gel formation.
92,98
The aggregation and phase behavior of Pluronics have been
extensively studied using several techniques including light scattering, NMR,
SANS, SAXS, DSC, microscopy, and rheology.
34,99−104
Pluronic F127, or Poloxamer 407, has previously been used for several appli-
cations in drug delivery. Its chemical formula is (PEO)
106
(PPO)
70
(PEO)
106
,itisa
nonionic surfactant, and in aqueous solution it can thermo-reversibly change from
its liquid phase into a gel structure at a gelation temperature T
G
, which depends
on its concentration. Aqueous solutions of Pluronic F127 are non-Newtonian
fluids with thermo-thickening and shear thinning properties.
26,99
The rheological
properties of Pluronic F127 solutions including the effect of salts on gelation as
well as its biocompatibility will be further discussed.
6.4.2.2 Rheological Characterization of Pluronic Solutions
Pluronic F127 solutions are thermo thickening materials. This means that upon
heating their viscosity increases gradually over a wide range of temperatures
until to their gel point, where the viscosity appears to increase dramatically.
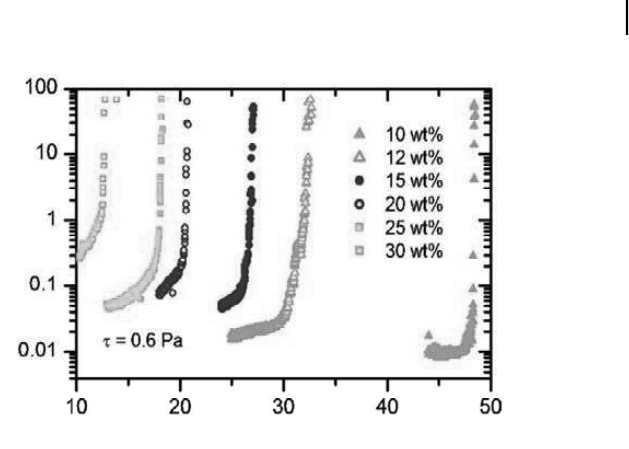
At higher concentration of Pluronic, gel forms at lower temperature. Stoeber
et al. have shown this behavior using rheometry for different concentrations
of Pluronic F127.
26
Continuous cone and plate vioscometry characterizes this
phase transition under conditions that are relevant for the application to fluids
streaming in microfluidic channels. This change in viscosity or phase change is
completely reversible and after one thermal cycle, the shear viscosity returns to its
SO13997_text.indd 128SO13997_text.indd 128 26/01/2011 3:50 PM26/01/2011 3:50 PM
Flow Control in Biomedical Microdevices using Thermally Responsive Fluids 121
dĞŵƉĞ ƌĂƚƵƌĞΣ
sŝƐĐŽƐŝƚLJWĂ Ɛ
Figure 6.1. Viscosity as a function of temperature for different concentrations of Pluronic
F127 in water from cone and plate viscometry at controlled shear stress (0.6 Pa s).
26
For color
reference, see page 265.
original value. The phase transition between liquid and gel occurs over a narrow
temperature range (0.5
◦
C) as shown in Fig. 6.1.
Pluronic F127 solutions also show pseudo plastic flow behavior (i.e. shear
thinning). Their viscosity occurs to decrease for increasing shear stress at a
constant temperature as previously shown using rheometry.
26
6.4.2.3 Effect of Ions on the Gelation Temperature of Pluronic Solutions
The effect of salt on micellization and also on the gelation temperature of Pluronic
F127 has previously been shown.
105−108
In general, a lowering of the micellization
and gelation temperature is observed upon addition of salt except for salts with
large polarizable anions, such as SCN
−
,andI
−
, where an initial increase in the
gelation temperature is often encountered. The effect is often discussed in terms
of “salting in” and “salting out”, of “structure-making” and ”structure-breaking”,
additives. The addition of salt is expected to further dehydrate PPO blocks and
induce some dehydration of the PEO blocks.
108
Almgren et al. have used a simple
but effective approach to qualitatively predict the effects of additives: type one,
those that increase the polarity difference between the solvent and the polymer
will favor phase separation and decrease the micellization temperature. Salts such
as NaCl, MgSO
4
and KF are depleted in the immediate vicinity of the polymer
with its low polarizability, and increase the polarity of water. Phase separation is
facilitated and so micellization occurs at a lower temperature. For salts of type
2, such as NaI or NaSCN on the other hand, the large, polarizable anion seems
to partition slightly in favor of the polymer, increasing its polarity and thereby
reducing the polarity difference. The polymer becomes more compatible with
SO13997_text.indd 129SO13997_text.indd 129 26/01/2011 3:50 PM26/01/2011 3:50 PM
Get Biomaterials for MEMS now with the O’Reilly learning platform.
O’Reilly members experience books, live events, courses curated by job role, and more from O’Reilly and nearly 200 top publishers.

