October 2017
Intermediate to advanced
816 pages
25h 46m
English
Consider a surface-reaction-limited irreversible isomerization
![]()
in which both A and B are adsorbed on the surface, and the rate law is
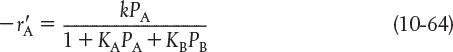
The specific reaction rate, k, will usually follow an Arrhenius temperature dependence and increase exponentially with temperature. However, the adsorption of all species on the surface is exothermic. Consequently, the higher the temperature, the smaller the adsorption equilibrium constant. That is, as the temperature increases, KA and KB decrease resulting in less ...