Symmetry
While everyone can appreciate the appearance of symmetry in an object, it is not so obvious how to classify it. The amide (1) is less symmetric than either ammonia or borane, but which of ammonia or borane – both clearly “symmetric” molecules – is the more symmetric ? In (1) the single N-H bond is clearly unique, but how do the three N-H bonds in ammonia behave ? Individually or as a group ? If as a group, how ? Does the different symmetry of borane mean that the three B-H bonds will behave differently from the three N-H bonds in ammonia ? Intuitively we would say “yes”, but can these differences be predicted ?
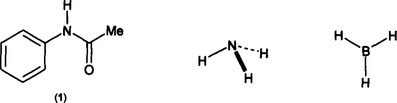
This opening chapter ...
Get Group Theory for Chemists, 2nd Edition now with the O’Reilly learning platform.
O’Reilly members experience books, live events, courses curated by job role, and more from O’Reilly and nearly 200 top publishers.

