Problems
- Calculate the variation of latent heat of fusion of ice with temperature from the following data:
Specific heat of water at 0°C = 1
Specific heat of ice at 0°C = 0.505
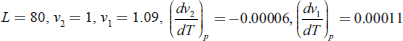
Ans.
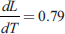
- At normal pressure, melting point of mercury is −39°C. Its density in solid state is 14.19 gm/cc and at liquid state 13.59 gm/cc and the latent heat of fusion is 2.33 cal/gm. Calculate the melting point of mercury at 1,000 atm.
Ans. 241.6 K
- The excess pressure inside a pressure cooker is maintained by a mass of 130 gm placed on the vapour exit tube having cross ...
Get Heat and Thermodynamics now with the O’Reilly learning platform.
O’Reilly members experience books, live events, courses curated by job role, and more from O’Reilly and nearly 200 top publishers.

