APPENDIX I
PHARMACEUTICAL AND BIOTECHNOLOGY ROYALTY RATES (NEW)
This compendium provides a readily accessible guide to royalty rate information in pharmaceuticals and biotechnology rights based on real-world transactions. Each section in this appendix is adapted from a reliable source, noted for each story, and the Appendix is considered to represent a comprehensive collection of technology pricing information.
Summarized in Exhibit I.1 is the royalty rate data discovered in the license agreements reported in Royalty Rates for Pharmaceuticals and Biotechnology, 7th edition. Only royalty rates, presented as a percent of sales, are included in the graph. For license agreements containing more than one rate, all rates are represented with equal weight in the graph.
The graph clearly shows the majority of royalty rates are in the mid to low single digits. Early-stage technology has typically commanded royalty rates and license fees that are relatively low. The risky and expensive nature of developing pharmaceutical and biotechnology coupled with the long lead time between discovery and commercialization provides significant downward pressure on royalty rates and license fees. However, when clinical trials and/or regulatory hurdles are passed, a successful commercialized therapy, available for licensing, commands double-digit royalty rates and enormous license fees.
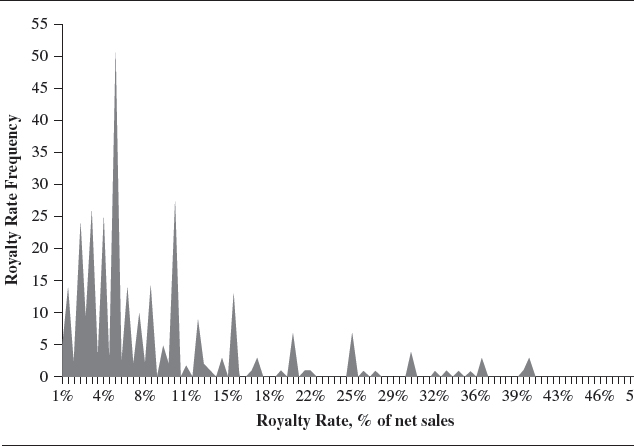
EXHIBIT I.1 ROYALTY ...
Get Intellectual Property: Valuation, Exploitation and Infringement Damages 2013 Cumulative Supplement, 11th Edition now with the O’Reilly learning platform.
O’Reilly members experience books, live events, courses curated by job role, and more from O’Reilly and nearly 200 top publishers.

