12
Electromotive Force and Oxidation–Reduction System
CHAPTER OBJECTIVES
12.2 Single Electrode Potential
12.3 Standard Electrode Potential
12.4 Measurement of Single Electrode Potential
12.7 Cell Potential or EMF of a Cell
12.8 Derivation of Nernst Equation (Concentration Dependence of Electrode Potential)
Electromotive force is the energy per unit charge that is converted reversibly from chemical, mechanical, or other forms of energy into electrical energy in a battery or dynamo.
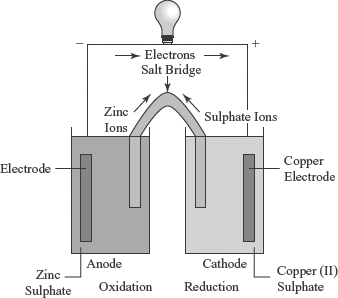
12.1 INTRODUCTION
We know that electric current can be used to bring about a chemical ...
Get Pharmaceutical Physical Chemistry: Theory and Practices now with the O’Reilly learning platform.
O’Reilly members experience books, live events, courses curated by job role, and more from O’Reilly and nearly 200 top publishers.

