Experiment 7
OBJECT
To find the strength of hydrochloric acid solution (approximately N/10) by titrating it against sodium hydroxide solution conductometrically.
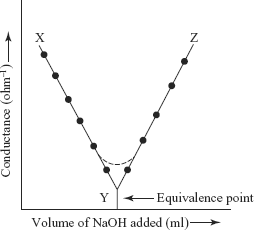
Figure Ex 7.1 Conductance curve of strong acid vs. strong base
THEORY
Conductometry can be used to detect the equivalence point (end point) of a titration. This method is based upon the measurement of conductance during the course of a titration. The Conductance varies differently before and after the equivalence point. This is due to the reason that electrical conductance of a solution depends upon the number of ions present and their ionic mobilities, i.e., speeds.
When conductance ...
Get Pharmaceutical Physical Chemistry: Theory and Practices now with the O’Reilly learning platform.
O’Reilly members experience books, live events, courses curated by job role, and more from O’Reilly and nearly 200 top publishers.

