Chapter 10
Electrochemical Sensors
Electrochemical sensors are devices that convert a chemical or physical property of a specific analyte into a measurable signal. The magnitude of this signal is desirably proportional to the concentration of the analyte. Certain biosensors are a subset of electrochemical sensors for the analysis of biological molecules.
Materials for their use in chemical sensors, their synthesis and applications have been profoundly documented, including topics such as molecular recognition, detection methods, design strategies, and important biological issues (1,2). Chemical and biological sensors can be classified into three major classes based on the principle of transduction of their response (3):
- Sensors with electrical transducers,
- Sensors with optical transducers, and
- Sensors with other transducers.
The classification of chemical sensors has been standardized (4).
10.1 Basic Principles
A sensor works in transforming an exposed analyte into a detectable physical signal. The analyte reacts with the active sensing material in some way in that the physical properties of the sensing material will change.
For example, in the case of poly(aniline) (PANI), carbon monoxide may react by a docking mechanism, which is shown in Figure 10.1.
Figure 10.1 Docking of carbon monoxide towards PANI (6)
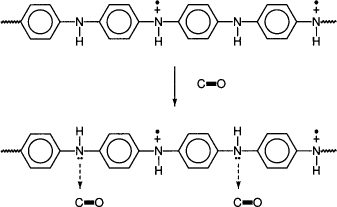
Carbon monoxide undergoes a complex because of its dipole moment with ...
Get Polymeric Sensors and Actuators now with the O’Reilly learning platform.
O’Reilly members experience books, live events, courses curated by job role, and more from O’Reilly and nearly 200 top publishers.

