October 2017
Intermediate to advanced
816 pages
25h 46m
English
1 For the limitations and for further explanation of these relationships, see, for example, K. Denbigh, The Principles of Chemical Equilibrium, 3rd ed. (Cambridge: Cambridge University Press, 1971), p. 138.
For the gas-phase reaction
![]()
1. The true (dimensionless) equilibrium constant
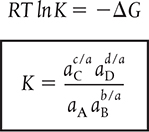
where ai is the activity of species i

where fi = fugacity of species i
= fugacity of species i at ...