4.12 Thermal Transpiration
Let us consider a gas enclosed in two vessels A and B at different temperatures T1 and T2, T1 >T2. The two vessels are separated by a heat-insulated porous plug (Fig. 4.33). Initially, the pressures are the same in both the vessels.
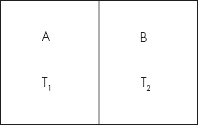
Fig. 4.33 Illustrating thermal transpiration
Let υ1 and υ2 be the densities of gas in the vessels. From the gas law p = υkT, we have υ1/υ2 = T2/T1.
The molecules from the vessel A moving to the vessel B are 1/4 υ1 ![]() and the molecules moving from the vessel B to the vessel A are 1/4 υ ...
and the molecules moving from the vessel B to the vessel A are 1/4 υ ...
Get Heat and Thermodynamics now with the O’Reilly learning platform.
O’Reilly members experience books, live events, courses curated by job role, and more from O’Reilly and nearly 200 top publishers.

