13. Semiconductors
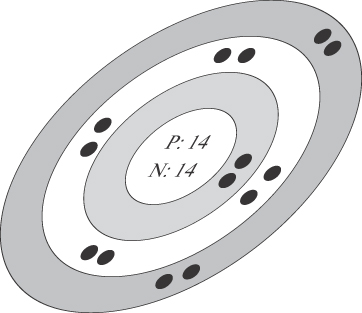
13.1. Electron Shells Revisited
In Chapter 1, we introduced the idea of electron shells around the nucleus of an atom. Atomic structures like copper, silver, and gold have a single electron in their outer (valence) shell and are good electrical conductors. Atomic structures like sulfur, whose outer shells are filled, are electrical insulators.1 Figure 13-1 shows a comparison of the atomic structures of copper and silicon. Silicon has four electrons in its outer shell.
1. This simplifies the distinction between conductors and insulators a little, but not much. For a good discussion of the difference, see http://en.wikibooks.org/wiki/Semiconductor_Electronics/Types_of_Materials ...
Get PCB Currents: How They Flow, How They React now with the O’Reilly learning platform.
O’Reilly members experience books, live events, courses curated by job role, and more from O’Reilly and nearly 200 top publishers.

