Chapter 6
Getting Dizzy with Spin
In This Chapter
- Discovering spin with the Stern-Gerlach experiment
- Looking at eigenstates and spin notation
- Understanding fermions and bosons
- Comparing the spin operators with angular momentum operators
- Working with spin ½ and Pauli matrices
Physicists have suggested that orbital angular momentum is not the only kind of angular momentum present in an atom — electrons could also have intrinsic built-in angular momentum. This kind of built-in angular momentum is called spin. Whether or not electrons actually spin will never be known — they're as close to point-like particles as you can come, without any apparent internal structure. Yet the fact remains that they have intrinsic angular momentum. And that's what this chapter is about — the intrinsic, built-in quantum mechanical spin of subatomic particles.
The Stern-Gerlach Experiment and the Case of the Missing Spot
The Stern-Gerlach experiment unexpectedly revealed the existence of spin back in 1922. Physicists Otto Stern and Walther Gerlach sent a beam of silver atoms through the poles of a magnet — whose magnetic field was in the z direction — as you can see in Figure 6-1.
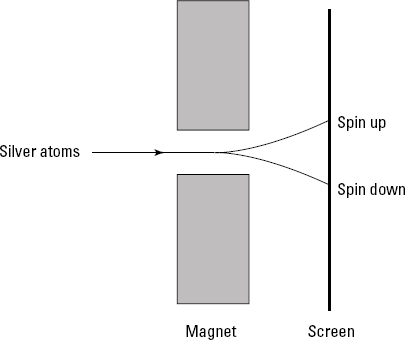
Figure 6-1: The Stern-Gerlach experiment.
Because 46 of silver's 47 electrons are arranged in a symmetrical cloud, they contribute nothing to the orbital angular momentum of the atom. The 47th electron can be in
- The 5s
Get Quantum Physics For Dummies, Revised Edition now with the O’Reilly learning platform.
O’Reilly members experience books, live events, courses curated by job role, and more from O’Reilly and nearly 200 top publishers.

