3Design Principles for Super Selectivity using Multivalent Interactions
Tine Curk1, Jure Dobnikar2, and Daan Frenkel1
1 Department of Chemistry, University of Cambridge, Lensfield Road, Cambridge CB2 1EW, UK
2 Institute of Physics & School of Physical Sciences, Chinese Academy of Sciences, Beijing, 100190, China
3.1 Introduction
Multivalent particles have the ability to form multiple bonds to a substrate. Hence, a multivalent interaction can be strong, even if the individual bonds are weak. However, much more interestingly, multivalency greatly increases the sensitivity of the particle–substrate interaction to external conditions, resulting in an ultra‐sensitive and highly non‐linear dependence of the binding strength on parameters such as temperature, pH or receptor concentration.
In this chapter we focus on super selectivity: the high sensitivity of the strength of multivalent binding to the number of accessible binding sites on the target surface (see the schematic drawing in Figure 3.1). For example, the docking of a multivalent particle on a cell surface can be very sensitive (super selective) to the concentration of the receptors to which the multiple ligands can bind.
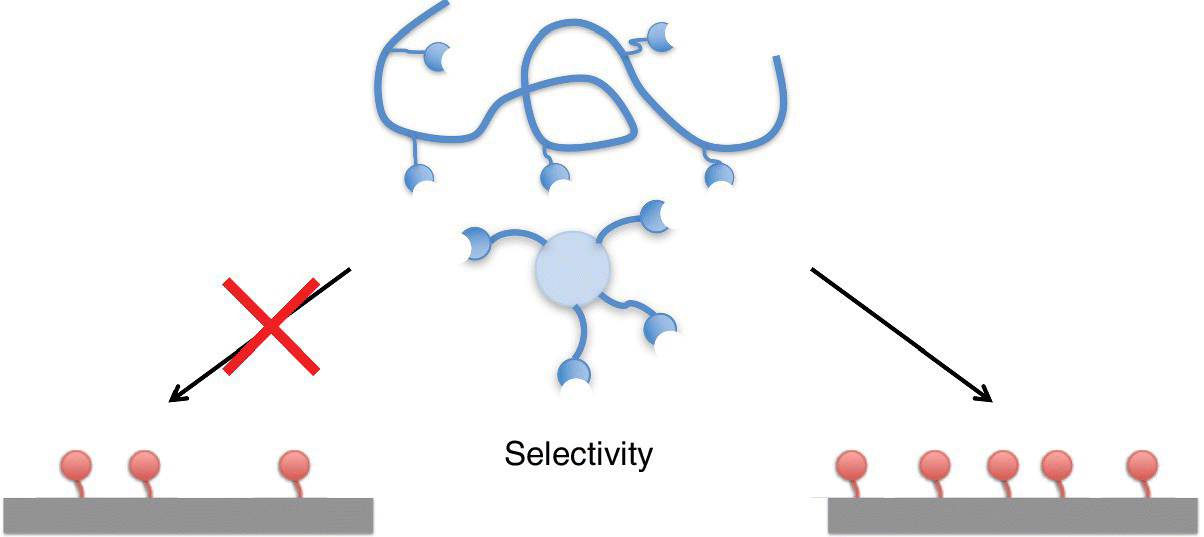
Figure 3.1 Selectivity denotes the ability of multivalent entities to distinguish between substrates depending on the surface density of binding sites.
We present a theoretical analysis of systems of ...
Get Multivalency now with the O’Reilly learning platform.
O’Reilly members experience books, live events, courses curated by job role, and more from O’Reilly and nearly 200 top publishers.

