6Cucurbit[n]uril‐Mediated Multiple Interactions
Zehuan Huang and Xi Zhang
Department of Chemistry, Tsinghua University, Beijing, 100084, China
6.1 Introduction to Cucurbit[n]uril Chemistry
The cucurbit[n]urils (CB[n]s) constitute a family of water‐soluble macrocyclic hosts, which have a hydrophobic cavity capable of binding one or two guest molecules (Figure 6.1) [1–3]. Three points contribute to the binding between CB[n] host and guest molecules [4–6]: (i) attraction between the host and guest; (ii) desolvation of the host cavity; and (iii) desolvation of the guest. In particular, the desolvation of the CB[n] host cavity releases high‐energy water trapped in the cavity which is regarded as a non‐classical hydrophobic effect as it has a favorable enthalpic signature. On account of their high affinities, CB[n]‐mediated host–guest interactions are of great interest in fabricating supramolecular systems with well‐defined composition and structure [7–11].
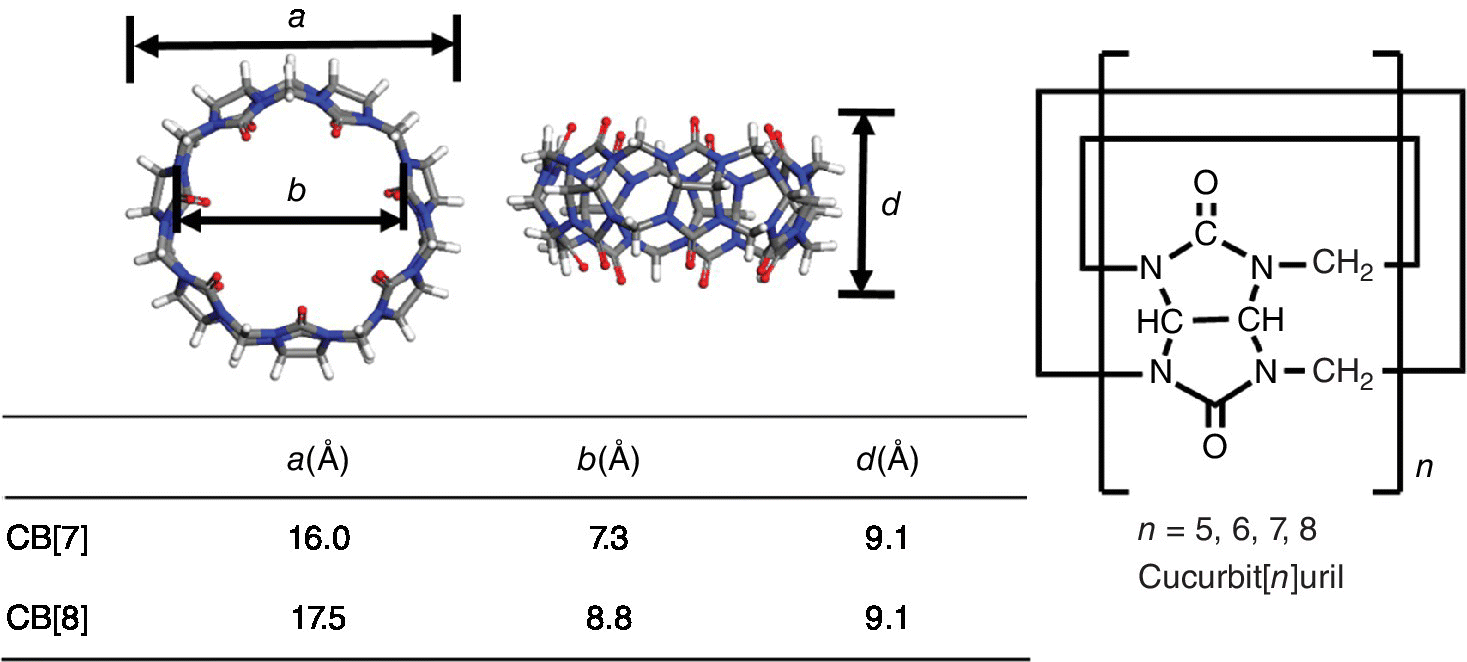
Figure 6.1 Molecular structures and detailed information of cucurbit[n]urils.
Among the cucurbit[n]uril familiy, cucurbit[8]uril (CB[8]) is a characteristic host which is capable of multiple binding with two guests (Figure 6.2). Based on whether binding different or the same guests, their complexation can be divided into heteroternary and homoternary complexations, respectively. CB[8]‐mediated heteroternary and homoternary ...
Get Multivalency now with the O’Reilly learning platform.
O’Reilly members experience books, live events, courses curated by job role, and more from O’Reilly and nearly 200 top publishers.

