14
Group — 13 (IIIA) B, Al, Ga, In, TI
Boron, Al, Ga, In and Tl are p — block elements and have three valence electrons (ns2np1).
Group in p — block = valence electrons + 10.
So, these elements belong to group — 13.
Electron configuration and core electrons
Table 14.1
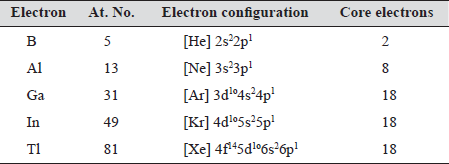
The difference in the number of core electrons and presence of 4f electrons in Tl has profound effect on the chemistry of these elements. Valence orbitals include p-orbitals. With the filling of p — orbitals, non-metallic property develops. Boron is thus a non-metal (slightly metallic). However, non-metallic nature decreases with increase in atomic number and Al, Ga, In and Tl ...
Get Basic Concepts of Inorganic Chemistry, 2nd Edition now with the O’Reilly learning platform.
O’Reilly members experience books, live events, courses curated by job role, and more from O’Reilly and nearly 200 top publishers.

