7.5 Condition for Liquefaction of Gases
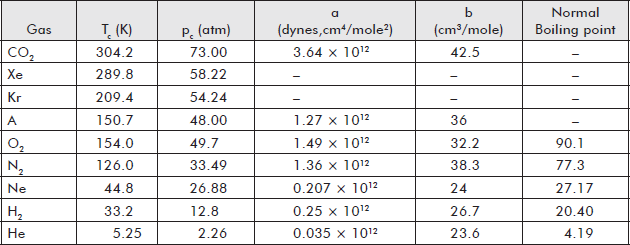
It appears from the characteristics of isothermals for different gases obtained from experiments of Amagat and others that for every gas there is a limiting value of temperature called the critical temperature above which a gas can never be liquefied by pressure alone, however high the pressure may be. So, the essential condition of liquefaction is that the temperature of the gas must be below its critical temperature. Table 7.1 shows the values of critical temperature and pressure of some gases.
Table 7.1 Some characteristic values of important gases
Below the critical temperature, a gas can be liquefied ...
Get Heat and Thermodynamics now with the O’Reilly learning platform.
O’Reilly members experience books, live events, courses curated by job role, and more from O’Reilly and nearly 200 top publishers.

