Solved Problems
1. Estimate the bond energy for the NaCl molecule as formed from sodium and chlorine atoms. The interionic equilibrium distance is 236 pm. Born constant is 8. The ionization energy of sodium is 5.14 eV and electron affinity of chlorine is 3.65 eV.
(Set-2–May 2004), (Set-4–Nov. 2004), (Set-2–May 2003)
Sol: The equilibrium separation of the ions (r0) = 236 pm = 236 × 10−12 m
The potential energy at this separation is:
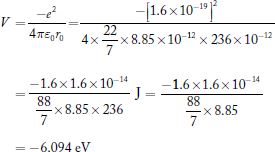
[energy released during the formation of molecule from ions]
Ionization energy of Na = 5.14 eV [energy supplied to remove an electron]
Electron affinity of chlorine = −3.65 eV
[energy released ...
Get Engineering Physics now with the O’Reilly learning platform.
O’Reilly members experience books, live events, courses curated by job role, and more from O’Reilly and nearly 200 top publishers.

